Introduction
Sickle cell disease affects a large population both nationally and globally. The disease is characterized by the presence of sickle hemoglobin, HbS, which polymerizes the red blood cell into a stiff, sickle shape upon deoxygenation. This polymerization causes several complications, most notably, vaso-occlusion. Voxelotor (Oxbryta, Global Blood Therapeutics) is a newly FDA approved therapeutic for the treatment of sickle cell disease that, when bound to HbS, maintains the oxy-Hb state and inhibits polymerization. Previous studies have demonstrated voxelotor's ability to improve the deformability of the sickle red blood cell (sRBC) via micropippeting and reduce viscosity under hypoxia through using a viscosmeter(Dufu et al, 2018), however its effect under dynamic flow conditions has yet to be explored. Microfluidic devices have served as useful tools to study sickle cell disease, allowing investigation under physiologic conditions of the rheological properties of the sRBC. In this experimental study we aim to examine voxelotor's effect on rheological properties of blood using a microfluidic platform that allows for direct observation of sickled blood flow in a physiologic relevant system.
Materials and Methods
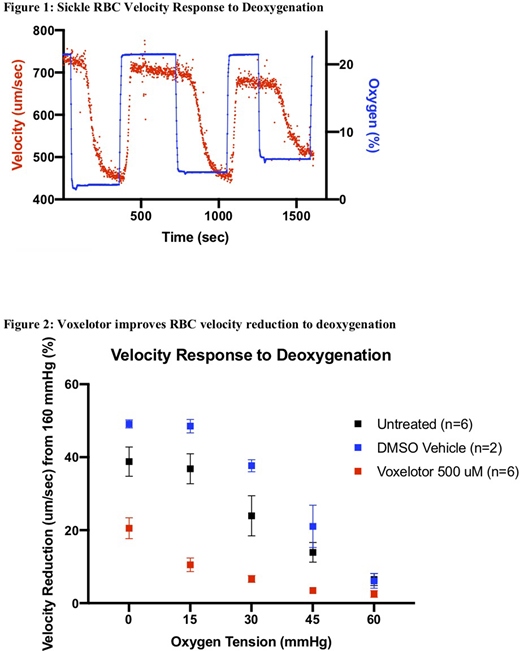
Whole blood was drawn from 6 patients with sickle cell disease (HbSS or HbSC) as a part of routine blood work under an IRB approved protocol. The cohort included both pediatric and adult patients both on and off hydroxyurea. A stock solution of voxelotor in DMSO (dimethylsulfoxide) was mixed and stored in -20C until use. Red blood cells (RBCs) were isolated using centrifugation and fixed to 25% hematocrit with saline. Voxelotor was added to the blood samples for a final concentration of 500 uM. Voxelotor treated samples were then incubated at 37C for one hour. An untreated, non-incubated aliquot from each patient sample was also obtained to serve a control. From two patient samples, a DMSO vehicle control was also incubated at 37C for one hour to serve as an additional control. Using an electronic pressure regulator, blood from each treatment was then driven through a microfluidic device at a constant pressure and was exposed to hypoxic conditions while RBC velocity data was collected. The microfluidic device design and fabrication in this experiment is described in previously published studies(Wood et al, 2012; Valdez et al, 2019). Briefly, a 3-layer microfluidic device constructed of polydimethylsiloxane (PDMS) consists of a blood, hydration, and gas layer. Saline is perfused through the hydration layer to prevent blood evaporation throughout the experiment. Oxygen gas is pushed through the gas layer, exposing flowing blood to a specific oxygen tension achieved using a mixing setup supplied by air and nitrogen tanks. A fiber optic sensor records oxygen tension within the gas layer throughout the experiment. Deoxygenation-oxygenation cycles were conducted using oxygen saturations from 0 to 21% (0 to 160mmHg pO2). With each deoxygenation cycle after 0%, oxygen saturations were up titrated in a stepwise fashion until oxygen-independent flow was observed. RBC velocity was evaluated by tracking cell movement in the microchannel using high frame-rate imaging and computation video processing.
Results and Conclusion
A reduction in velocity occurs when sickle RBCs are exposed to deoxygenated conditions as seen in one sample example tracing in figure 1. However, the addition of voxelotor at 500 uM improved the blood flow response to deoxygenation, as RBCs treated with voxelotor had a reduction in velocity change compared to vehicle control and untreated samples when exposed to hypoxic conditions as low as 0 mmgHg oxygen (figure 2). Additionally, voxelotor treated samples began to experience oxygen-independent velocity at lower oxygen tensions compared to the controls. By inhibiting polymerization, voxelotor improves sensitivity of sickle RBC blood flow response in hypoxic conditions. While polymerization is one aspect of sickle cell disease, we would like to explore further effects of voxelotor on other aspects of the understood pathophysiology of the disease such as effects on adhesion in future experiments.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal